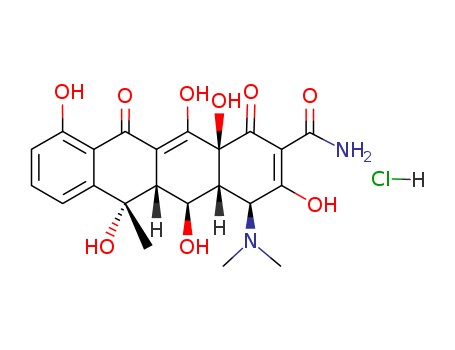
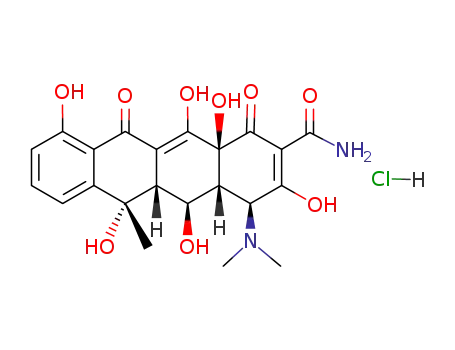
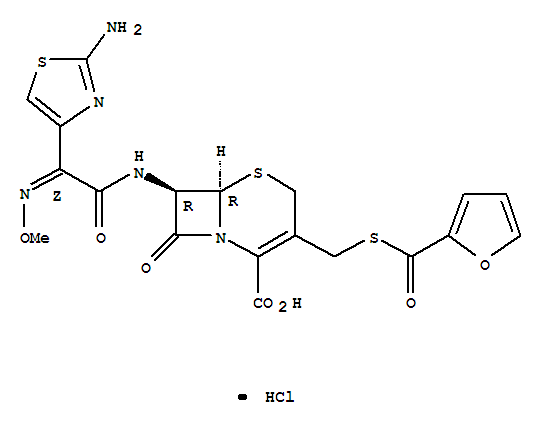
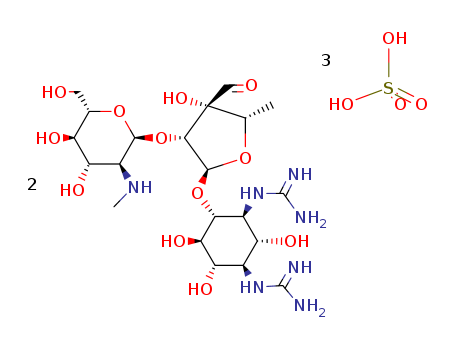
Buy High Quality Quality Manufacturer Supply Oxytetracycline hcl 2058-46-0 with Efficient Transportation
- Molecular Formula:C22H25ClN2O9
- Molecular Weight:496.901
- Appearance/Colour:Yellow crystalline solid
- Vapor Pressure:2.17E-30mmHg at 25°C
- Melting Point:180 °C
- Boiling Point:845.6 °C at 760 mmHg
- Flash Point:465.2 °C
- PSA:201.85000
- Density:==
- LogP:0.25870
Oxytetracycline hydrochloride(Cas 2058-46-0) Usage
|
Manufacturing Process
|
The pH was adjusted to 7.0 with sodium hydroxide and calcium carbonate was added at the rate of 1 g/l. 500 ml portions of the above medium were added to Fernbach flasks which were then sterilized at 121°C for 30 minutes. Upon cooling, the flasks were inoculated with a suspension of the growth of s. rimosus obtained from the surface of beef lactose agar slants, and the flasks were shaken for 4 days at 28°C on a rotary shaker having a displacement of 2" at an rpm of 200. At the end of this period the broth was found to contain 640 C.D.U/ml and 400 chloramphenicol units/ml. The mycelium was separated from the broth by filtration and the latter was adjusted to pH 9.0. The antibiotic was extracted from the broth with n-butanol, and when the ultraviolet absorption spectrum was observed on the butanol solution of the antibiotic, peaks in the absorption curve were found at 385 and 270 millimicrons.
|
|
Therapeutic Function
|
Antibiotic
|
|
Air & Water Reactions
|
Oxytetracycline hydrochloride is hygroscopic. Water soluble. Undergoes slow hydrolysis in the presence of water. Concentrated aqueous solutions at neutral pH hydrolyze on standing.
|
|
Reactivity Profile
|
Oxytetracycline hydrochloride is sensitive to light. Oxytetracycline hydrochloride may be unstable at temperatures above 77° F. Oxytetracycline hydrochloride darkens on exposure to sunlight or to moist air above 194° F. Concentrated aqueous solutions at neutral pH hydrolyze on standing. Oxytetracycline hydrochloride undergoes hydrolysis in the presence of water. Oxytetracycline hydrochloride may be incompatible with alkalis.
|
|
Fire Hazard
|
Flash point data for Oxytetracycline hydrochloride are not available. Oxytetracycline hydrochloride is probably combustible.
|
|
Brand name
|
Terramycin (Pfizer).
|
|
General Description
|
Early in 1950, Finlay et al.182 reported the isolation of oxytetracycline(Terramycin) from S. rimosus. This compoundwas soon identified as a chemical analog of chlortetracycline that showed similar antibiotic properties. The structureof oxytetracycline was elucidated by Hochstein et al.183 andthis work provided the basis for the confirmation of thestructure of the other tetracyclines.Oxytetracycline hydrochloride is a pale yellow, bitter,crystalline compound. The amphoteric base is only slightlysoluble in water and slightly soluble in alcohol. It is odorlessand stable in air but darkens on exposure to strong sunlight.The hydrochloride salt is a stable yellow powder that ismore bitter than the free base. It is much more soluble inwater, 1 g dissolving in 2 mL, and more soluble in alcoholthan the free base. Both compounds are inactivated rapidlyby alkali hydroxides and by acid solutions below pH 2. Bothforms of oxytetracycline are absorbed rapidly and equallywell from the digestive tract, so the only real advantage thefree base offers over the hydrochloride salt is that it is lessbitter. Oxytetracycline hydrochloride is also used for parenteraladministration (intravenously and intramuscularly).
|
InChI:InChI=1/C22H24N2O9.ClH/c1-21(32)7-5-4-6-8(25)9(7)15(26)10-12(21)17(28)13-14(24(2)3)16(27)11(20(23)31)19(30)22(13,33)18(10)29;/h4-6,12-14,17,25,27-29,32-33H,1-3H3,(H2,23,31);1H/t12-,13-,14+,17+,21-,22+;/m1./s1
2058-46-0 Relevant articles
Process and intermediate for the purification of oxytetracycline
-
, (2008/06/13)
The invention relates to a new process f...
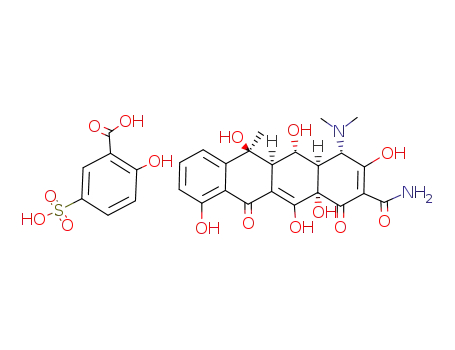
Interaction between Oxytetracycline Sulphosalicylate and Calcium Chloride
Issar, M. M.,Mahadevan, P. R.
, p. 257 - 258 (2007/10/02)
Oxytetracycline sulphosalicylate (OTc-SA...
2058-46-0 Process route
-
- 61068-97-1
oxytetracycline 5-sulphosalicylate
-
- 2058-46-0
oxytetracycline hydrochloride
Conditions
| Conditions |
Yield |
|
With calcium chloride; In methanol; at 60 ℃;
|
80% |
-
- 2058-46-0
oxytetracycline hydrochloride
2058-46-0 Upstream products
2058-46-0 Downstream products
-
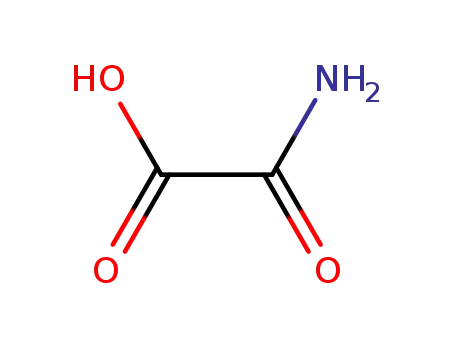
471-47-6
oxamic acid
-
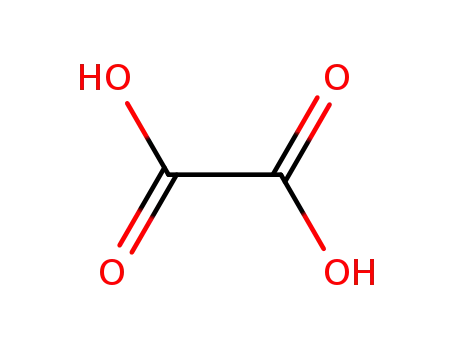
144-62-7
oxalic acid
-
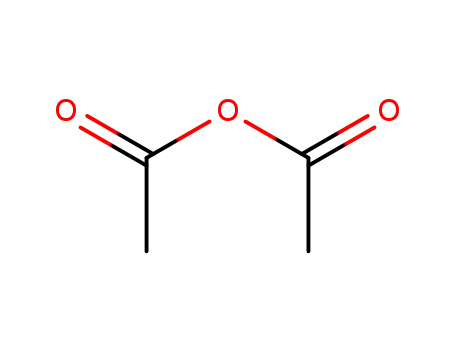
108-24-7
acetic anhydride
-
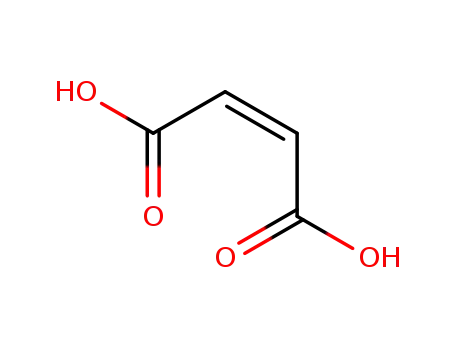
110-16-7
maleic acid
 English
English 中文
中文
 English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego
English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego