|
Sulfonamide drugs
|
Sulfa drugs have a relative broad antimicrobial spectrum and have certain inhibitory effect against most Gram-positive bacteria and gram-negative bacteria. However, different sulfonamide drugs have different extent of antibacterial efficacy. With the wide application of sulfonamide drugs, it will also be easy to lead to resistant strains; in particular the opportunity of resistant Staphylococcus aureus will be even higher than other kinds of resistant strains. According to the absorption situations after oral administration, the sulfonamide drugs can be classified into two categories, the first category is easily absorbed sulfonamide drugs, which is characterized by rapid absorption. Generally, their plasma concentrations can reach peak at 2-4 hours after the administration (5 hour for long-acting drugs). This kind of sulfonamide drugs are mainly used for systemic infections. Another kind of drugs is hardly or poorly absorbed after oral administration with most of them being excreted through the intestinal tract and is mainly used for treating intestinal infections. Sulfamethoxazole is a broad-spectrum antibiotic with particularly strong efficacy on Staphylococcus aureus and Escherichia coli and can be used for the treatment of urinary tract infections and fowl cholera. Sulfamethoxazole belongs to a systemic, moderate sulfonamide drug. It can compete with PABA through taking effect on the bacterial in vivo dihydrofolate synthase, preventing the bacterial synthesis of dihydrofolate and thereby inhibiting the bacterial growth and reproduction. It, together with other three kinds of sulfonamide drugs: sulfadiazine, sulfisoxazole and trisulfapyrimidine are currently excellent drugs for the treatment of nocardiosis. It has a half-life of 10 to 12 hours and can be partially acetylated. This product, although has the number of required medication be less than sulfisoxazole (2 times per day instead of four times), but its acetylated metabolites has a low solubility in the urine so the likelihood of large crystals formation in urine is bit higher. Patients should maintain enough hydrated conditions (the daily adult urine output should be not less than 1,500ml). When being used in combination with the synergist trimethoprim, its antibacterial activity can get significantly enhanced. Sulfamethoxazole compound (sulfamethoxazole/trimethoprim namely SMZ/TMP) can often give better efficacy than monotherapy (see dihydrofolate reductase inhibitors). Clinically it is commonly used for the treatment of urinary tract infections, respiratory tract infections, typhoid, and salmonella infections, Pneumocystis carinii echinococcosis and nocardiosis. It can also be used for preventing meningococcal meningitis. Some clinicians advocate use minocycline, erythromycin or ampicillin in combination with sulfamethoxazole to treat such infections. However, there has been no clinical data for proving that combination therapy is superior to single administration of sulfonamide. Sulfamethoxazole compound (TMP/SMZ), minocycline (Minocin) and amikacin can also be used for the treatment of nocardia infection.
|
|
Mechanism of action
|
It is known that bacterial can synthesize thymidine, purine and finally synthesize DNA and need folic acid derivatives, tetrahydrofolate as cofactors. Most bacterial cells can’t allow the penetration of folic acid and instead needs the synthesis through aminobenzoic acid (PABA). Sulfonamide has a similar structure to PABA and thus can competitively inhibit the synthesis of the direct precursor of dihydrofolate, dihy-dropteroic acid through the reaction between PABA and pteridine. Mammalian cells, instead, is not inhibited since they require preformed folate and is not capable of synthesizing this product. Treatment concentration of sulfonamide drug sulfamethoxazole is primarily bacteriostatic agents. However, when the bacteria is grown in the medium containing purine, amino aicd but very low concentration of thymine, the sulfonamide drugs can produce a bactericidal effect, that is, "thymine lethal defect". This bactericidal effect has been demonstrated in human blood and urine (Then and Angehrn, 1973 A and B; see also Pratt and Fekety, 1986). The effect of sulfonamide drug-induced inhibition of bacterial cell growth can be reversed upon supplement in vitro of some substances (such as thymidine, purines, methionine and serine) to the growth medium. This may have important clinical significance. Because the pus generated by cells destruction may contain a large amount of these substances. It is therefore, upon purulent infection, the effect of the drug may be inhibited probably due to the presence of such substances. Furthermore, for the in vitro susceptibility testing, the medium can’t contain PABA for which even in trace amount, the test results can be disturbed. The above information is edited by the lookchem of Dai Xiongfeng.
|
|
Production method
|
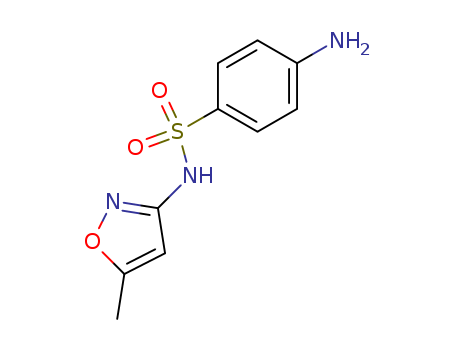
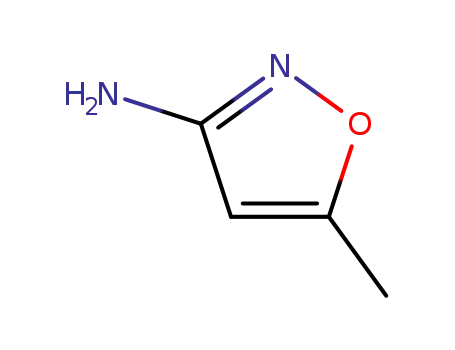
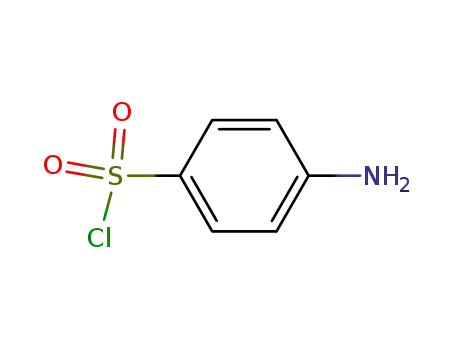
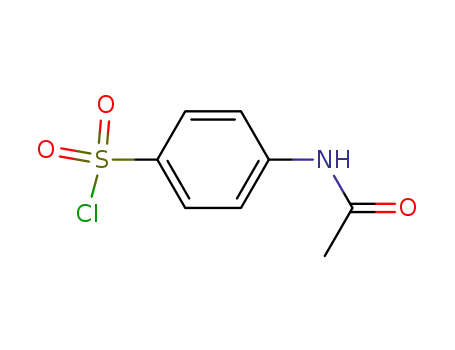
It can be produced from 5-methyl-isobutyl-3-carboxamide via degradation, condensation and hydrolysis successively. Use 5-methyl-isobutyl-3-carboxamide as raw materials, it is degraded into 5-methyl-isoxazol-3 amine under the action of sodium hypochlorite solution, then condense with para-acetamidophenoxymethyl chloride to generate 3-(p-acetamide benzenesulfonamido)-5-methylisoxazole with further hydrolysis under alkaline conditions to give 3-(p-amino benzenesulfonamido)-5-methylisoxazole.
|
|
Antimicrobial activity
|
The intrinsic activity is similar to that of sulfadiazine.
|
|
Air & Water Reactions
|
Insoluble in water.
|
|
Fire Hazard
|
Flash point data for Sulfamethoxazole are not available but Sulfamethoxazole is probably non-flammable.
|
|
Pharmaceutical Applications
|
This is the sulfonamide component of co-trimoxazole. It is slightly soluble in water.
|
|
Pharmacokinetics
|
Oral absorption: 85% Cmax 800 mg oral: c.50 mg/L after 3–6 h Plasma half-life: 6–20 h Volume of distribution: 12–18 L Plasma protein binding: 65% Penetration of extravascular sites, including the CSF, is good. It crosses the placenta and achieves levels in breast milk of about 10% of the simultaneous plasma concentration. It is extensively metabolized, but about 30% of the dose is excreted unchanged in urine so that high concentrations are achieved.
|
|
Side effects
|
Unwanted effects are those common to sulfonamides. In addition, benign intracranial hypertension has been reported in children. Most side effects of co-trimoxazole are thought to be attributable to the sulfonamide component.
|
|
Safety Profile
|
Moderately toxic by ingestion and intraperitoneal routes. Questionable carcinogen with experimental tumorigenic data. When heated to decomposition it emits very toxic fumes of NOx and SOx.
|
|
Synthesis
|
Sulfamethoxazole, N1 -(5-methyl-3-isoxazolyl)sulfanilamide (33.1.20), is synthesized by a completely analogous scheme, except by using 3-amino-5-methylisox�azol as the heterocyclic component.
|
|
Brand Name(s) in US
|
Gantanol
|
|
Definition
|
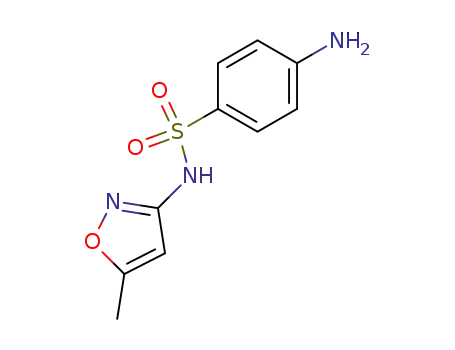
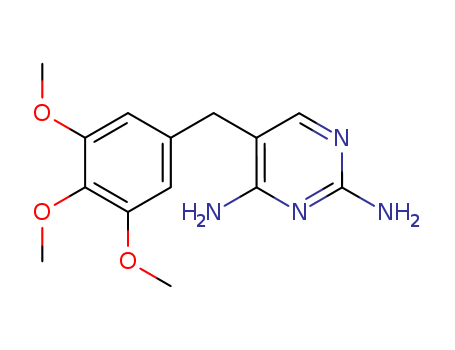
ChEBI: An isoxazole (1,2-oxazole) compound having a methyl substituent at the 5-position and a 4-aminobenzenesulfonamido group at the 3-position.
|
|
Brand name
|
Gantanol (Roche); Urobak (Shionogi).
|
|
General Description
|
Sulfamethoxazole’s plasma half-life is 11 hours. Sulfamethoxazole is a sulfonamide drug closely relatedto sulfisoxazole in chemical structure and antimicrobial activity.It occurs as a tasteless, odorless, almost white crystallinepowder. The solubility of sulfamethoxazole in the pHrange of 5.5 to 7.4 is slightly lower than that of sulfisoxazole but higher than that of sulfadiazine, sulfamerazine, or sulfamethazine.Following oral administration, sulfamethoxazole is notabsorbed as completely or as rapidly as sulfisoxazole, andits peak blood level is only about 50% as high.
|
 English
English 中文
中文
 English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego
English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego