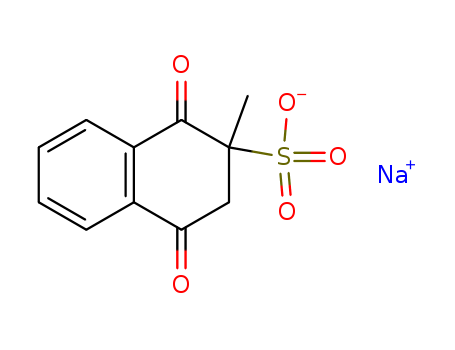
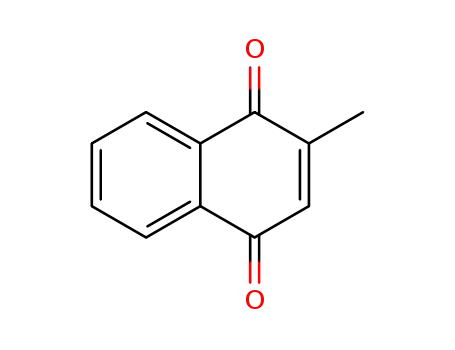
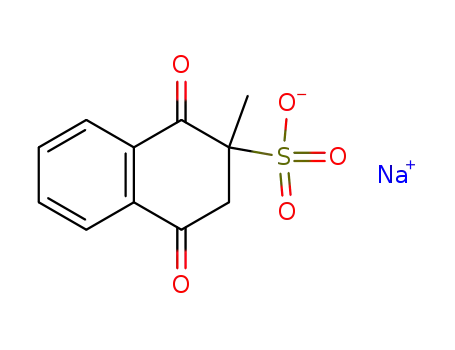
Top Purity Reputable Factory Supply Menadione sodium bisulfite 130-37-0 with Safe Delivery
- Molecular Formula:C11H9NaO5S
- Molecular Weight:276.245
- Appearance/Colour:Crystalline powder
- Melting Point:121-124 °C
- PSA:99.72000
- LogP:1.84040
Menadione sodium bisulfite(Cas 130-37-0) Usage
|
Definition
|
ChEBI: An organic sodium salt that is the monosodium salt of menadione sulfonate. A synthetic naphthoquinone without the isoprenoid side chain and biological activity, but can be converted to active vitamin K2, menaquinone, after alkylation i vivo.
|
|
Manufacturing Process
|
The 2-naphthalenesulfonic acid, 1,2,3,4-tetrahydro-2-methyl-1,4-dioxo-, sodium salt, trihydrate can be prepared by mixing the 2-methyl-1,4- naphthoquinone with the bisulphite salt in the presence of water. Ordinarily gentle warming of the aqueous mixture is preferred to facilitate solution. The mixture of 2-methyl-1,4-naphthoquinone (250 mg; 1 molar equivalent); sodium bisulphite (149 mg; 1 molar equivalent); distilled water (250 ml) or 2- methyl-1,4-naphthoquinone (250 mg; 1 molar equivalent); potassium bisulphate (349 mg; 2 molar equivalent); distilled water 250 ml may be used. These examples representing preferred ratios of ingredients are merely illustrative and are not to be interpreted as limiting.The bisulphite addition compounds have been found to be stable in sunlight and also to be heat stable. Tests, for example, carried out in ampoules have shown aqueous solutions of the compounds not to be decomposed after exposure to a month's sunlight, while other tests have shown the solutions of such compounds to retain their original potency (a) when stored in an oven at 60°C for 15 days or (b) when sterilized at 15 pounds for 0.5 hour in an autoclave at about 122°C. These properties emphasize the radical differences between the stable salts and the properties of 2-methyl-1,4-naphthoquinone, the characteristic instability of which is illustrated by its sensitivity, i. e., decomposition, when exposed to light.The bisulphite addition compounds have a vitamin K activity equal to that of the 2-methyl-1,4-naphthoquinone contained in the molecule. The compounds, although suitable for oral administration, are particularly adaptable in aqueous solution for parenteral administration in the treatment of hemorrhagic conditions.
|
|
Brand name
|
Hykinone (Abbott); Klotogen (Abbott).
|
|
Therapeutic Function
|
Prothrombogenic vitamin
|
|
Biochem/physiol Actions
|
Menadione sodium bisulfite is a water-soluble form of menadione, which belongs to the Vitamin K class of compounds. These are necessary for the biosynthesis of prothrombin and other blood clotting factors. Menadione is a prothrombogenic compound and is used as a model quinone in cell culture and in vivo investigations. Menadione has been shown to affect gap-junctional intercellular communication by mediation of tyrosine phosphorylation. Menadione has demonstrated cytotoxic activity against a variety of cell lines and can induce apoptosis in cultured cells, such as osteoclasts and osteoblasts, via elevation of peroxide and superoxide radical levels.An HPLC method for detection of menadione sodium bisulfite in multivitamin formulations has been published. A chemiluminescence assay for menadione sodium bisulfite in pharmaceutical preparations and biological fluids has been reported.
|
InChI:InChI=1/C11H10O5S.Na/c1-11(17(14,15)16)6-9(12)7-4-2-3-5-8(7)10(11)13;/h2-5H,6H2,1H3,(H,14,15,16);/q;+1/p-1
130-37-0 Relevant articles
New route to vicasol, a water-soluble form of vitamin K3
Matveev,Odyakov,Zhizhina
, p. 469 - 472 (2001)
A procedure was suggested for preparing ...
Preparation method of menadione sodium hydrogen sulfite
-
Paragraph 0059; 0082-0087, (2021/07/31)
The invention provides a preparation met...
Green preparation method of menadione sodium bisulfite
-
Page/Page column 0078-0083, (2021/07/31)
The invention provides a preparation met...
A method for continuous preparation of menadione sodium bisulfite (by machine translation)
-
Paragraph 0041; 0042, (2017/03/17)
The invention discloses a tubular method...
Method for producing 2-methyl-1,4-naphthoquinone
-
Page/Page column 5-6, (2008/06/13)
[PROBLEM TO BE SOLVED]: To provide a met...
130-37-0 Process route
-
- 58-27-5,34524-96-4
menadione
Conditions
| Conditions |
Yield |
|
With sodium hydrogensulfite; In ethanol; water; at 40 - 45 ℃; for 4h; Solvent; Temperature;
|
97.5% |
|
With sodium hydrogensulfite; In ethanol; water; at 40 - 45 ℃; for 4h; Solvent; Temperature;
|
97.5% |
|
With sodium hydrogensulfite; In ethanol; water; at 75 ℃; Temperature;
|
78% |
|
With sodium hydrogensulfite; In chloroform; water; at 20 - 40 ℃; for 2h;
|
|
-
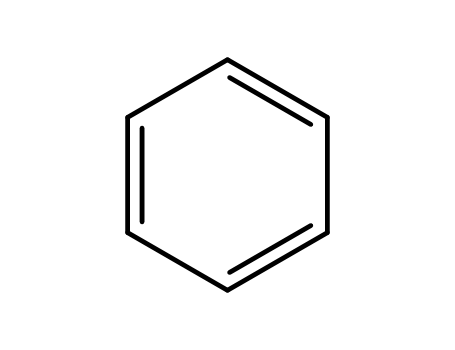
- 71-43-2,26181-88-4,54682-86-9,13967-78-7,174973-66-1
benzene
Conditions
| Conditions |
Yield |
|
Multi-step reaction with 4 steps
1: aluminum (III) chloride / 17 h / 40 - 82 °C
2: sodium hydroxide; dihydrogen peroxide; hydrogen bromide / dichloromethane; water / 25 - 30 °C
3: air / isopropyl alcohol / 5 h / 90 °C
4: sodium hydrogensulfite / water; ethanol / 4 h / 40 - 45 °C
With aluminum (III) chloride; hydrogen bromide; dihydrogen peroxide; sodium hydrogensulfite; sodium hydroxide; In ethanol; dichloromethane; water; isopropyl alcohol;
|
|
130-37-0 Upstream products
-
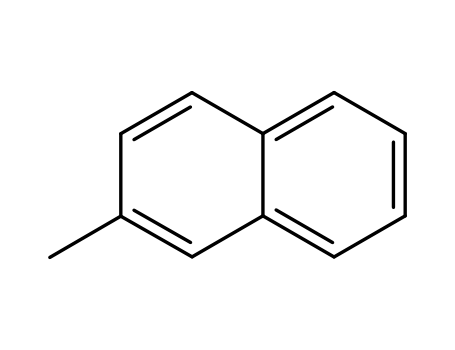
58-27-5
menadione
-
91-57-6
2-Methylnaphthalene
-
71-43-2
benzene
-
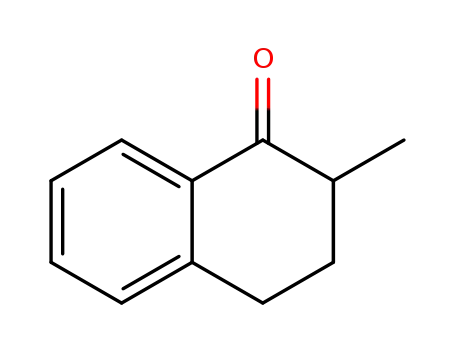
1590-08-5
2-methyl-1-tetralone
130-37-0 Downstream products
 English
English 中文
中文
 English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego
English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego