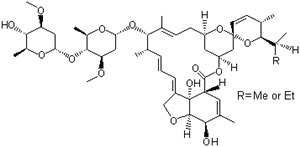
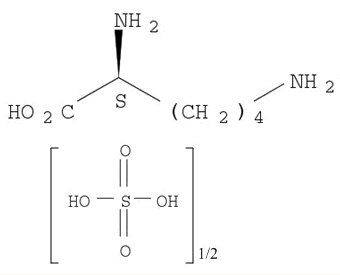
Reputable Manufacturer Supply Best Quality Abamectin 71751-41-2 with Cheap Price
- Molecular Formula:C48H72O14;C47H70O14
- Molecular Weight:(873.09);(859.06)
- Appearance/Colour:white powder
- Vapor Pressure:0mmHg at 25°C
- Melting Point:0-155 °C
- Refractive Index:1.571
- Boiling Point:940.912 °C at 760 mmHg
- Flash Point:268.073 °C
- PSA:170.06000
- Density:1.244 g/cm3
- LogP:5.62500
Abamectin(Cas 71751-41-2) Usage
|
Bio-pesticides
|
Abamectin, is a member of the avermectin family and is a natural fermentation product of soil dwelling actinomycete Streptomyces avermitilis. Abamectin differs from ivermectin, the popular member of the avermectin family, by a double bond between carbons 22 and 25. Abamectin is a kind of 16-membered ring macrolide compound which was first developed by the Kitasato University in Japan and Merck Company (United States). It has insecticidal, acaricidal, and nematicidal activity. According to the pundits’ prediction, the 21st century will be the century of biological pesticides. It is reported that the European bio-pesticides sales increased from 100 million dollar (1997) to 160 million dollars in 2004. Abamectin is the most popular and highly competitive novel biological pesticide in currently bio-pesticide market.
|
|
Uses
|
Abamectin is used by the agricultural community to control insects and mites on a large range of crops such as: citrus, pears, alfalfa, nut trees, cotton, vegetables, and ornamental. When abamectin is applied to crops and orchards, it is absorbed by the foliage and affects the insect when it eats the leaves. Abamectin, widely used as a veterinary anthelmintic, medicine against a variety of animal parasites and insects, can runoff from the sites of application and becomes an aquatic pollutant.
|
|
Analysis Methods
|
High Performance Liquid Chromatography.
|
|
Reactivity Profile
|
A lactone.
|
|
Hazard
|
A poison by ingestion. Moderately toxic by inhalation and skin contact.
|
|
Mechanism of Action
|
Abamectin acts on both glutamate-gated chloride channels (GluCls) and 纬-aminobutyric acid (GABA)-gated chloride channels in arthropods, leading to paralysis of the target organisms.
|
|
Definition
|
Any of a group of broad spectrum antiparasitic antibiotics produced by the actinomycete, Streptomyces avermitilis.
|
|
Who Evaluation
|
Evaluation year: 1998
|
InChI:InChI=1/C48H72O14/c1-11-25(2)43-28(5)17-18-47(62-43)23-34-20-33(61-47)16-15-27(4)42(26(3)13-12-14-32-24-55-45-40(49)29(6)19-35(46(51)58-34)48(32,45)52)59-39-22-37(54-10)44(31(8)57-39)60-38-21-36(53-9)41(50)30(7)56-38/h12-15,17-19,25-26,28,30-31,33-45,49-50,52H,11,16,20-24H2,1-10H3/b13-12+,27-15-,32-14-/t25-,26-,28-,30-,31-,33+,34-,35-,36-,37-,38-,39-,40+,41-,42-,43+,44-,45+,47+,48+/m0/s1
71751-41-2 Relevant articles
Abamectin in the aquatic environment
Tatjana Tišler & Nevenka Kožuh Eržen
Ecotoxicology, Volume 15, pages 495–502, (2006)
The aim of this study was to identify the toxicity of abamectin on bacteria, algae, daphnids, and fish. An extremely high toxicity of avermectin to the survival and reproduction of Daphnia magna was observed in 21-day exposure tests. Zebrafish and the algae Scenedesmus subspicatus are less sensitive to avermectin. The compound is expected to have adverse effects on the aquatic environment due to its high toxicity, even at very low concentrations, to daphnids and to fish.
Environmental aspects of abamectin use in crop protection
PG Wislocki, LS Grosso, RA Dybas
Ivermectin and abamectin, 1989
Abamectin undergoes rapid photolysis under a number of different conditions. Photolysis occurs when abamectin is exposed to light in water, on soil particles, or as a thin film on either …
Toxicity of abamectin and doramectin to soil invertebrates
L Kolar, NK Eržen, L Hogerwerf, CAM van Gestel
Environmental Pollution, 2008
This study aimed at determining the toxicity of avermectins to soil invertebrates in soil and in faeces from recently treated sheep. Abamectin was more toxic than doramectin.
 English
English 中文
中文
 English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego
English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego